Procedure Writing
Standard Operating Procedure (SOP) Writing Guide (with Word+Excel templates)
The following tutorial describes how to write Standard Operating Procedures. It includes detailed writing instructions, samples, and links to professional MS Word templates.
Use these Standard Operating Procedure (SOPs) templates to plan, structure, write, and maintain your procedure documents.
We designed these templates so they are flexible enough to work across business, technical, academic, and other industries. As experienced procedure writers, we’ve used these templates on consultancy projects in the USA, UK, and China for some of the largest IT firms and government agencies.
Learn more about these templates
1.1 What is a Standard Operating Procedure?
A Standard Operating Procedure is a specific type of document that describes how to perform an action, typically by following a set of instructions or steps.
Standard Operating Procedures, also known as SOPs, are frequently used in military, medical, and emergency environments.
SOPs are part of the overall quality management system and provides individuals with the information to perform an action correctly, which ensures quality and integrity in the final product.
1.2 What are the Benefits of a SOP?
The benefit that Standard Operating Procedures offers is that it provides employees with easy to follow instructions resulting in less errors and greater efficiency.
1.3 What is the Purpose of a SOP?
According to the FAO website:
“The purpose of a SOP is to carry out the operations correctly and always in the same manner. A SOP should be available at the place where the work is done”.
Use SOPs to document a frequently recurring process that an organization performs or follows.
SOPs document how to perform an activity in a consistent manner in conformance with technical and quality system requirements. For example, a SOP might describe the steps to maintain, calibrate, and use equipment.
In general, SOPs are specific to an organization to assist them maintain their quality control and quality assurance processes, and ensure compliance with governmental or financial regulations.
Management need to review and re-enforce SOPs on a regular basis to ensure relevance.
In addition, the SOP owners should share the most current copy of SOPs with employees and made available on an easy-to-access website. Likewise, out of date SOPs should be archived and removed from public access to avoid data contamination; for example, staff following an out-dated set of instructions.
1.4 Why are Standard Operating Procedures important?
SOPs provide reliable direction when performing a commonly-repeated task.
For example?
Ever tried to assemble a set of shelves?
At some point, you’ll look at the instructions manual to see how it fits together. Maybe you don’t read it line by line, but you look at it now and then for direction.
Actually, even if you don’t read it, you can look at the diagrams. The point is that, with the manual, you have some frame of reference.
SOPs offer the same benefit.
That’s the benefit of having a written set of procedures. You can see how it is supposed to fit together. When you get lost, you can read, line by line, each instruction.
1.5 Why Write Standard Operating Procedures?
If you work in any environment where staff need to follow instructions, you should write up a set of procedures.
The lack of standard operating procedures means that each employee will interpret how to perform the task as they understand it.
While this might works, it also means that staff will take more time that necessary to complete a task. Likewise, if they skip a step – and still complete the task – they might think it has been completed correctly. Only later will the error arise.
1.5.1 Standard operating procedures in lab work
The importance of standard operating procedures in laboratory work is that it gives staff a set of instructions, a checklist to follow, which improves the quality of the final product.
Indeed, the benefits of sops in pharmacy is that you avoid rework and deliver a faster, more reliable product. It’s also more compliant.
1.5.2 I don’t have time to write a standard operating procedure
Yes, it takes time to write standard operating procedures but it pays off in the long run.
High quality SOPs improve the quality of the deliverable and allow you to quickly identify errors or omissions in the product development lifecycle.
Learn more about these templates
1.6 What are the pros and cons of SOPs?
The benefits of a SOP is that it reduces effort, improves credibility, and ensures a quality product.
Develop SOPs to minimize deviations, promote quality by consistently implementing a procedure, and indicate compliance. Likewise, use SOPs to minimize miscommunications and address safety concerns.
Finally, quality inspectors can use SOPs as checklists to audit procedures.
1.7 SOP Writing Guidelines
What writing style should you use to write a SOP?
Use the following guidelines to write standard operating procedures:
1.7.1 Purpose
Introduce the purpose of the standard operating procedure. What will the reader have achieved after they have completed this task.
1.7.2 Steps
Write the SOPs in a concise, step-by-step, easy-to-read format.
1.7.3 Accuracy
Write each step so that it describes how to perform one single action. Do not merge or blend more than one action in a single step. If there is an option, then split the steps into sub-steps.
1.7.4 Active voice
Use the active voice. Tell the reader what to do. Avoid disguising the action using passive voice.
1.7.5 Concise
Use short words. Aim to help the reader understand the task at a single reading.
Avoid adjectives and flowery speech. Likewise, avoid acronyms or jargon.
1.7.6 Positive Language
Use positive language to describe how to perform the task. Guide the reader to perform the task.
1.7.7 Diagrams
If necessary, provide a flow chart to illustrate the process.

Learn more about these templates
2. Writing SOPs
How to Prepare SOP?
Use the following checklist to prepare your standard operating procedures
- Identify the procedures or processes that need to be documented.
- Identify who has the writing skills to write the documents.
- Identify who has the subject matter expertise to contribute, verify, and review the SOPs.
- Create a team to write, edit, review, and publish the SOPs.
- Create and share a template to write the SOPs. Share this with all team members.
- Write the SOPs with sufficient detail so that user can reproduce the procedure when unsupervised.
- Publish new SOPs. Archive redundant SOPs.
2.1 Reviewing and Approving SOPs
Who should review SOPs?
Follow the steps in the Quality Management Plan to write, review, and approve the SOPs.
The SOP review process requires input from individuals with sufficient knowledge of the subject matter to determine if the SOP is:
- Accurate – the procedure is easy to follow, accurate, and avoids any ambiguity.
- Complete – the procedure includes all the information required to complete the task. No critical steps, prerequisites, or warning messages have been overlooked.
- Subject matter experts – people with dep skills in specific areas – should review the procedure, then work with the actual procedure writer to ensure the document is updated and correct before approval.
As part of the review process, make sure to test the SOP before signing it off.
In general, the quality assurance manager will review and approve each SOP.
2.2 Frequency of SOP Reviews
How often should you review your SOPs?
It depends… but it’s recommended to review your SOPs at least once a year.
Make sure to schedule SOP reviews on a periodic basis, e.g. every 1-2 years, to ensure that the policies and procedures remain current, and determine if new SOPs are needed.
Why?
Because of updates to the underlying procedures which needs to be capture in the documentation.
Likewise, it’s good practice to schedule annual reviews to ensure any gaps, omissions, or errors have not crept into the quality documentation.
- Update the SOPs when procedures are changed.
- Modify only the pertinent section of the SOP.
- Highlight the change date/revision number in the Table of Contents and document control.
- Add the review date added to each SOP that has been reviewed.
- Remove and archive invalid SOP.
- Streamline the review to encourage timely reviews.
- Indicate the frequency of SOP reviews in the Quality Management Plan.
- Indicate the individual(s) responsible for ensuring that SOPs are current.
2.3 SOP Writing Checklist
Should you use checklists to write SOPs?
While checklists are an important part of the SOP, you need to give equal attention to other parts. For example, make sure to include any prerequisites or warnings to the reader.
However, when it comes to the actual writing of the SOP, a checklist identifies the sequence to follow when performing the procedure.
Use checklist to:
- Identify the starting point in a procedure.
- Identify the sequence.
- Identify related procedures.
- Identify conditions.
- Identify the ending point in a procedure.
Remember: the checklist is not the SOP, but a part of the SOP.

Learn more about these templates
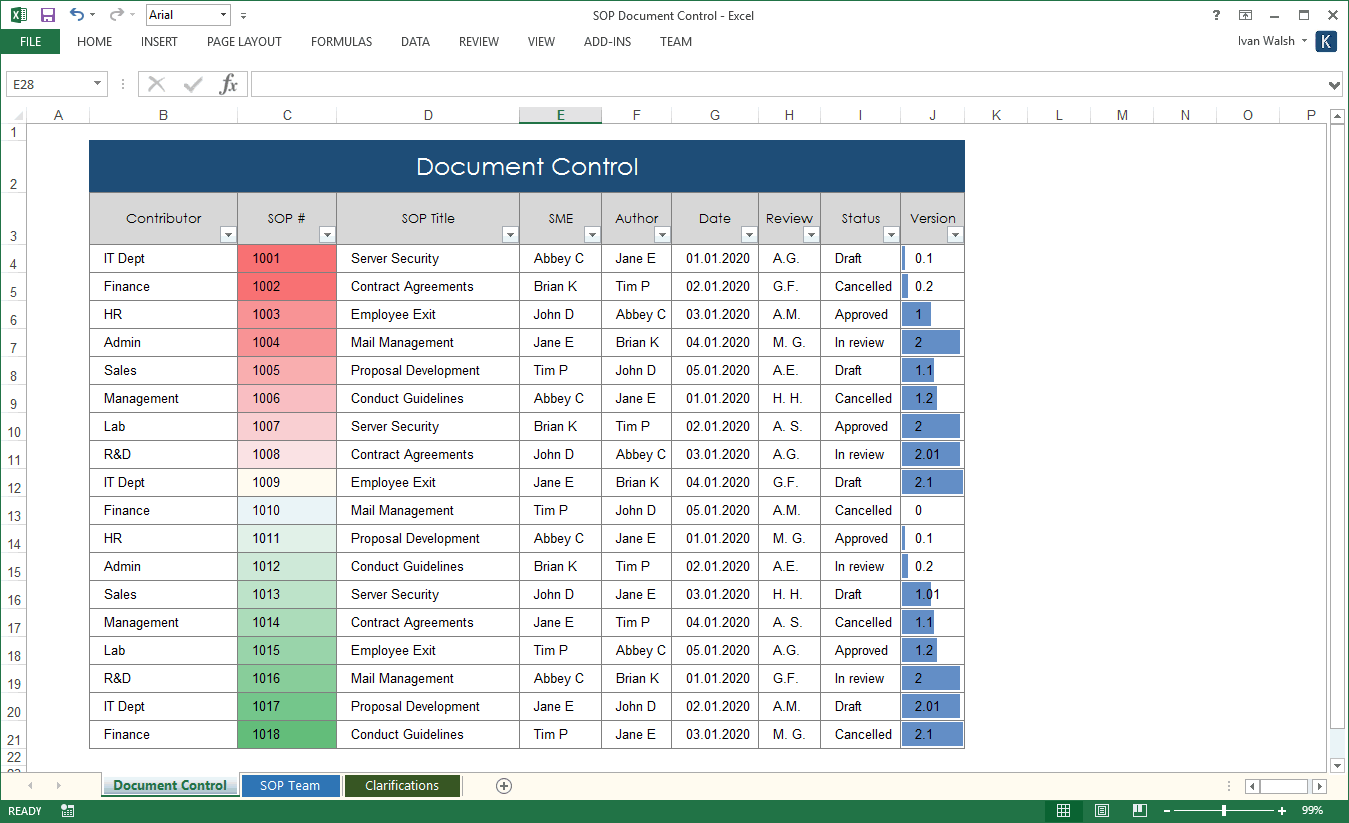
2.4 SOP Document Control
What document control guidelines do SOPs need?
Follow these guidelines to ensure SOPs are documented correctly.
- Develop a numbering system to systematically identify and label your SOPs
- Describe the document control numbering in the Quality Management Plan
- Apply document notation to each page in the SOP
- Include a short title and identification (ID) number to serve as a reference designation.
- Include a revision number to identify the most recent review.
- Include the date to identify when the SOP was updated.
- Include the number of pages on all pages.
- Add the document control notation in the upper right-hand corner of each document page following the title page.
Title/Identifier:
Revision Number:
Date:
Page X of Y
2.5 SOP Document Tracking
So, how do you track your SOPs?
Like those beautiful flowers in your garden, you need order or things get out of control. Suddenly, you have weeds.
To track your SOP documents:
- Maintain a master list of all SOPs.
- Indicate the SOP number, version number, date of issuance, title, author, status, division, branch, section, and historical information regarding previous versions.
- Delegate authorized individuals to maintain the current quality-related SOPs.
- Use the document management system to send notifications when new SOPs are issued.
2.6 SOP Archiving
Need to archive your SOPs?
Use the following guidelines to archive out of date SOPs.
- In the Quality Management Plan, indicate the individual(s) responsible for assuring that only the current version is used.
- Identify where outdated versions are to be archived.
- Identify how obsolete versions are to be archived to avoid data contamination as well as making the documents available for historical data review.
Learn more about these templates
2.7 SOP Document Format
What format should you use to document your SOPs?
Use the following guidelines to format your SOPs:
Organize your SOPs to make them easy to document and update.
Where possible, break the information into logical steps to avoid a long list.
Title Page
On the first or cover page of each SOP, include the following information:
- The title must identify the procedure
- The SOP identification (ID) number
- The date of issue and/or revision
- The name of the agency, division, and/or branch to which the SOP applies.
Table of Contents
Include a Table of Contents if the SOP is more than a few pages, and to help the reader find critical information faster.

2.8 Main Text of SOP
Use the following guidelines to write the main text in the SOP:
- Briefly describe the purpose of the procedure, including any regulatory information or standards that are appropriate to the SOP.
- Indicate the scope of what is covered.
- Define any specialized or unusual terms in a glossary of terms.
- Denote the sequence in which to perform procedures, divided into significant sections; e.g., equipment needed, personnel qualifications, and safety considerations.
- Describe QA and QC activities for that procedure
- List any cited or significant references.
- Write SOPs using language that is easy to understand, presented in a format that clearly describes the steps in order.
- Use diagrams and flow charts help to break up long sections of text and to briefly summarize a series of steps for the reader.
- Attach any appropriate information, e.g., references to other SOPs.
2.9 Guidelines for Technical SOPs
Write technical SOPs to instruct users to:
- Perform specific analytical methods in the laboratory or field
- Collect a sample in order to preserve the sample integrity
- Conduct an assessment.
In addition, technical SOPs cover activities related to data processing, valuation, verification and validation, modeling, risk assessment, and auditing.
In general, include the following information in technical SOPs
- Title Page
- Table of Contents
- Procedures – Include the following topics in technical SOPs. Not all may apply to every procedure.
- Scope and Applicability – describe the purpose of the process or procedure, and any limits to the use of the procedure
- Summary – briefly summarize the procedure
- Definitions – identify any acronyms, abbreviations, or specialized terms used
- Health & Safety Warnings – indicate any operations that could result in injury; explain what will happen if the procedure is not followed correctly
- Cautions – indicates activities that could result in equipment damage, degradation of samples, or possible invalidation of results
- Interferences – describe any component that may interfere with the accuracy of the final product
- Qualifications/Responsibilities – identify the minimal user experience to complete the task; cite any applicable requirements
- Equipment – list and specify equipment, materials, standards etc.
- Procedure – identify all pertinent steps, in the correct order, and the materials needed to accomplish the procedure.
- Quality Control – follow QC activities to verify the quality and consistency of the work. Describe the procedures to report QC data and results.
- Reference – reference documents or procedures that interface with the SOP.
Learn more about these templates
Download Standard Operating Procedure (SOPs) templates
Use these SOP templates to plan, structure, write, and maintain your procedure documents.
We designed these templates so they are flexible enough to work across business, technical, academic, and other industries. As experienced procedure writers, we’ve used these templates on consultancy projects in the USA, UK, and China for some of the largest IT firms and government agencies.


